HIV vaccine trial in Africa halted after disappointing initial results
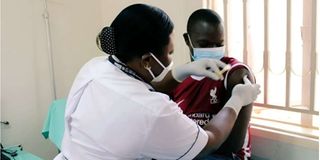
The first PrEPVacc volunteer participant receives an injection at the launch of the launch of the PrePVacc Trial at the Masaka site of the MRC/UVRI and LSHTM Uganda Research Unit, on December 15, 2020. PHOTO | PrEPVacc Investigators
What you need to know:
- The trial's findings, however, underscore the ongoing challenges in developing an effective HIV vaccine and the need for continued research and exploration of alternative prevention strategies.
Dar es Salaam. A large-scale HIV vaccine trial conducted across Eastern and Southern Africa from 2020 to 2024 has concluded without success.
The PrEPVacc trial, which included sites in Tanzania, Uganda and South Africa, failed to demonstrate that either of the two experimental vaccine regimens could reduce HIV infections among the study population of 1,512 high-risk adults.
The results revealed yesterday from the PrEPVacc project statement are particularly significant for Tanzania, where the need for effective HIV prevention measures remains critical.
Mbeya and Dar es Salaam were among the four sites where the trial took place; others were Masaka in Uganda and Durban in South Africa.
It enrolled healthy adults aged 18–40 years, all of whom engaged in behaviours that heightened their vulnerability to HIV.
The participant demographics included 87 percent women and 13 percent men. While the Tanzanian sites in Mbeya and Dar es Salaam enrolled only women, the others included both men and women.
PrEPVacc’s Chief Investigator, Prof Pontiano Kaleebu, said the vaccine questions posed in the trial have been answered, and what is clear is that these vaccines won’t be taken any further.
“The results have been surprising and disappointing as well. But that is science. It has been a very good study, following the highest international standards. Now, we must advance because the world needs to have choices in its HIV prevention toolbox. A vaccine against HIV remains a critically sought-after and important part of that toolbox,” he said.
PrEPVacc’s Trial director, Dr Eugene Ruzagira, who presented the results to the AIDS 2024 conference yesterday, said vaccine trials were halted in November 2023 as soon as it was evident that the vaccines were ineffective.
“The positive news for our communities is that repeated risk reduction counselling and the use of proven HIV prevention tools help people navigate their risks better.
The number of new infections has fallen throughout the six years we have been monitoring the HIV incidence in each study community,” he said.
According to their statement, PrEPVacc tested two different combinations of HIV vaccines against a placebo. One regimen combined a DNA vaccine with a protein vaccine, while the other combined the same DNA vaccine with a modified non-dividing virus vector and a protein-based vaccine.
The vaccination schedule included four injection visits over approximately six months, with a fourth visit a year after enrollment.
However, challenges associated with the Covid-19 pandemic, which affected recruitment and vaccine supply, led to changes in the trial, focusing only on the DNA and protein vaccine and placebo groups by June 2022.
The primary analysis showed that among those who received at least three injections of the DNA and protein vaccine combination, 11 out of 532 participants acquired HIV, yielding an incidence rate of 1.73 infections per 100 person-years.
In comparison, 3 out of 523 participants who received the placebo acquired HIV, with an incidence rate of 0.48 infections per 100 person-years.
For the modified virus and protein vaccine combination, 9 out of 244 participants acquired HIV, with an incidence rate of 2.38 infections per 100 person-years, compared to 2 out of 251 participants in the placebo group with an incidence rate of 0.51 infections per 100 person-years.
“These results mean that neither vaccine combination offered any protective effect, nor the researchers say that the confidence intervals around the hazard ratios are so wide they cannot draw a definitive conclusion about what the higher number of infections in the vaccine arms means,” they concluded.
PrEPVacc Project Lead based at the Medical Research Council clinical trials unit at University College London,Prof Sheena McCormack, noted that the imbalance between infections in the vaccine groups and the placebo was unexpected and could not be explained.
“We suspect chance but cannot rule out the possibility the result is plausible, so it is clear we need to continue to support the participants and provide HIV testing to monitor the trend,” she said.
“The total number of infections in the trial was much smaller than we expected, which is good news, and I hope this reflects what is happening in the wider community. We had seen a decline in infections from 2018 in the Registration Cohort, but even so, the placebo incidence rate was unusually low. As this impacts both vaccine results, it adds to our sense of uncertainty in the comparisons.”